
Three Types of Endometriosis
Endometriosis Types
There are three main types of endometriosis, each with its symptoms and treatments. The three categories are superficial peritoneal, ovarian cysts, and deep infiltrating. Here’s a brief overview of each type:
Superficial Peritoneal Endometriosis: This type involves growths on the surface layer of tissue lining the abdomen (the peritoneum). These growths usually appear as small spots or lesions. They can cause pain during periods or intercourse. Treatment typically includes surgery, medication, or lifestyle modification, depending on the patient’s preference and symptoms.
Deep Infiltrating Endometriosis: This type involves growths penetrating deeper into tissues and organs. It can cause extensive scarring, severe pelvic pain, and difficulty getting pregnant. Treatment for this type may include surgical removal of the endometriosis lesions, medical therapy, lifestyle modification, and physical therapy. Deep infiltrating endometriosis can impact any organ in the body, such as the bowel, bladder, diaphragm, etc.
Ovarian Cysts: Also known as “chocolate cysts” because they contain dark-colored tissue and blood. These cysts form on or near the ovaries and can cause pain. Treatment for ovarian cysts may include the removal of the cyst through surgery.
Endometriosis is a complex condition with many potential treatments available. In general, there are three types of endometriosis which include superficial endometriosis, deep infiltrating endometriosis, and endometrioma. The type of endometriosis might impact your treatment plan. It’s important to talk with your doctor about your specific case so that you can better understand your diagnosis and treatment options in the future. If you are experiencing any concerning symptoms related to endometriosis, such as pelvic pain or painful sex, make sure you get checked out by a qualified healthcare professional who can provide further guidance on managing your condition effectively!

Understanding the Different Stages of Endometriosis
Endometriosis is a condition that affects nearly 10% of women in the United States. It occurs when tissue similar to the lining of the uterus grows outside the uterus, causing pain and other symptoms. The severity of endometriosis can vary from person to person and impact the treatment strategy. So it’s essential to know the different stages of endometriosis and how they affect your body. Here we review the most common classification of endometriosis introduced by ASRM (American Society for Reproductive Medicine.)
Stage 1 Endometriosis (Minimal): superficial small lesions or implants outside the uterus or near pelvic organs.
Stage 2 Endometriosis (Mild): encompasses more and deeper implants in the pelvic area.
Stage 3 Endometriosis (Moderate): This is a deep infiltrating endometriosis stage. In this stage, many deep implants are in the pelvic area and other body parts. There are small cysts on one or both ovaries. Some adhesions are present in the abdomen and pelvis.
Stage 4 Endometriosis (Severe): A deep infiltrating endometriosis stage. This is the most severe stage. There are many deep lesions, large cysts on one or both ovaries, and dense abdomen and pelvis adhesions.
Knowing what stage of endometriosis you have can help you understand your condition better and guide your doctor in developing an appropriate treatment plan. If you suspect you have endometriosis, make sure to speak with your gynecologist for proper diagnosis and treatment. No matter what stage you are at with your endometriosis diagnosis, good management will help improve your quality of life and reduce pain and long-term complications from this condition.

Can Endometriosis Be Cured Completely?
Endometriosis is a common condition that affects 1 in 10 women between 15 and 55 years old. It occurs when tissue similar to the lining of the uterus grows outside the uterus. Endometriosis can cause painful periods and sex, abdominal pain, and fertility issues. But what exactly is endometriosis, and can it be cured? Let’s take a closer look.
Table of contents
What Causes Endometriosis?
The exact cause of endometriosis is unknown, but several theories exist about how it develops. One theory suggests that during menstruation, some of the uterine linings flow back through the fallopian tubes and into the abdomen, where it implants and begins to grow. Another theory suggests that stem cells present in the body can transform into endometriosis tissue.
Can Endometriosis Be Cured Completely?
Unfortunately, no—endometriosis cannot be cured entirely at this time. However, there are treatments available to alleviate symptoms. These treatments include hormonal medications or surgery, depending on your condition’s severity and preferences. Hormonal medications such as birth control pills or hormone-releasing IUDs may help reduce pain and stop endometriosis growth from progressing further by stopping ovulation and changing hormone levels in your body. Surgery may also be a choice, especially if you have severe symptoms that are not responding to routine treatments. Surgery is also more desirable with deeply infiltrated lesions or cysts on your organs, such as your ovaries or intestines.
Endometriosis affects millions of women worldwide. Although not curable, you can manage this disease with proper surgery, medical care, and lifestyle plans. Lifestyle steps such as maintaining a healthy diet, exercising regularly, reducing stress levels, and avoiding certain foods known to worsen your symptoms can be helpful. Although there is currently no cure for endometriosis, many treatment options exist that can help minimize symptoms. These treatment options can help you lead a near-normal life and improve pain or fertility issues. If you suspect you have endometriosis, talk to your doctor about available treatments for managing your condition today!

Endometriosis Dictionary:
Endometriosis is a disease that affects at least one in ten women and produces pain and subfertility. The pain can be related to the menstrual cycle or be related to specific activities like pain during and after sex. Pain medications may help quality of life but the diagnosis of endometriosis and effective treatment is rooted in surgical excision and some hormone therapy, which may be mainstream or integrative or holistic in some cases. There is a lexicon of terms that comes with understanding endometriosis including the following:
| Term | Definition | Phonetic Spelling |
|---|---|---|
| Abdominal Cavity | The space within the abdomen that houses the intestines, liver, and other organs. | /ab·doh·mi·nuhl kav·i·tee/ |
| Ablation | The removal or destruction of tissue using heat, laser, or other methods. | /uh·blay·shun/ |
| Adhesions | Bands of scar tissue that bind organs together. | /ad·hee·zhuhnz/ |
| Adenomyosis | A condition in which endometrial-like tissue exists within and grows into the uterine muscle wall. | /ad·uh·noh·my·oh·sis/ |
| Amenorrhea | The absence of menstruation. | /ay·men·uh·ree·uh/ |
| Analgesic | A medication that reduces or eliminates pain. | /an·uhl·jee·zik/ |
| Anovulation | The absence of ovulation. | /an·ov·yuh·lay·shun/ |
| Aromatase Inhibitors | Drugs that inhibit the enzyme aromatase, reducing estrogen levels. | /uh·roh·muh·tayz in·hib·i·terz/ |
| Biopsy | A medical test involving the extraction of sample cells or tissues for examination. | /bye·op·see/ |
| Bilateral Oophorectomy | Surgical removal of both ovaries. | /bye·lat·uh·ruhl oh·uh·fuh·rek·tuh·mee/ |
| Catamenial Pneumothorax | A rare condition where air leaks into the space between the lungs and chest wall during menstruation. | /kat·uh·mee·nee·uhl noo·moh·thor·aks/ |
| Cervix | The lower part of the uterus that opens into the vagina. | /sur·viks/ |
| Chocolate Cyst | Ovarian cysts filled with old blood, also known as endometriomas. | /chaw·klit sist/ |
| CO2 Laser | A laser used in surgical procedures to cut or vaporize tissue. | /see·oh·too lay·zer/ |
| Cul-de-sac | The area between the uterus and the rectum where endometriosis commonly occurs. | /kull·duh·sak/ |
| Deep Infiltrating Endometriosis (DIE) | Severe form of endometriosis that invades deeper tissues. | /deep in·fil·tray·ting en·doh·mee·tree·oh·sis/ |
| Dyschezia | Painful bowel movements, often associated with endometriosis. | /dis·kee·zee·uh/ |
| Dysmenorrhea | Painful menstruation. | /dis·men·uh·ree·uh/ |
| Dyspareunia | Painful intercourse. | /dis·puh·roo·nee·uh/ |
| Endocrinologist | A doctor who specializes in the endocrine system, which regulates hormones. | /en·doh·kri·nah·luh·jist/ |
| Endometrioma | A type of cyst formed when endometrial-like tissue grows in the ovaries. | /en·do·me·tree·oh·muh/ |
| Endometriotic Lesions | Areas of endometrial-like tissue growth outside the uterus. | /en·doh·mee·tree·ot·ik lee·zhunz/ |
| Endometrium | The inner lining of the uterus that thickens and sheds during the menstrual cycle. | /en·do·mee·tree·um/ |
| Endometriosis | A condition where tissue similar to the lining inside the uterus grows outside it. | /en·do·mee·tree·oh·sis/ |
| Endovaginal Ultrasound | An ultrasound test performed via the vagina to get a closer look at the reproductive organs. | /en·doh·vaj·in·uhl ul·truh·sownd/ |
| Estrogen | A hormone that plays a key role in the development of female reproductive tissues and secondary sexual characteristics. | /es·truh·jen/ |
| Excision Surgery | A surgical procedure to cut out endometriosis tissue. | /ek·si·zhun sur·juh·ree/ |
| Fallopian Tubes | Tubes that carry eggs from the ovaries to the uterus. | /fuh·loh·pee·uhn toobs/ |
| Follicle-Stimulating Hormone (FSH) | A hormone involved in the development of eggs in women and sperm in men. | /fol·i·kul stim·yuh·lay·ting hor·mohn/ |
| Gonadotropin | Hormones that stimulate the activity of the gonads (ovaries and testes). | /goh·nad·oh·troh·pin/ |
| Gonadotropin-releasing Hormone (GnRH) Agonists/Antagonists | Drugs that reduce estrogen production by affecting the pituitary gland. | /goh·nad·oh·troh·pin ree·lees·ing hor·mohn ag·oh·nist/ |
| Hormone Replacement Therapy (HRT) | A treatment used to relieve symptoms of menopause by replenishing estrogen and progesterone. | /hor·mohn ree·plays·muhnt thair·uh·pee/ |
| Hysterectomy | Surgical removal of the uterus. | /his·tuh·rek·tuh·mee/ |
| Hysterosalpingography | An X-ray procedure to examine the inside of the uterus and fallopian tubes. | /his·ter·oh·sal·pin·goh·grah·fee/ |
| Implantation | The process by which a fertilized egg attaches to the lining of the uterus. | /im·plan·tay·shun/ |
| In Vitro Fertilization (IVF) | A procedure in which eggs are fertilized by sperm outside the body and then implanted in the uterus. | /in vee·troh fur·tuh·luh·zay·shun/ |
| Infertility | The inability to conceive after one year of unprotected intercourse. | /in·fur·til·i·tee/ |
| Interstitial Cystitis | A chronic bladder condition causing bladder pain and frequent, urgent urination. | /in·ter·stish·uhl si·sty·tis/ |
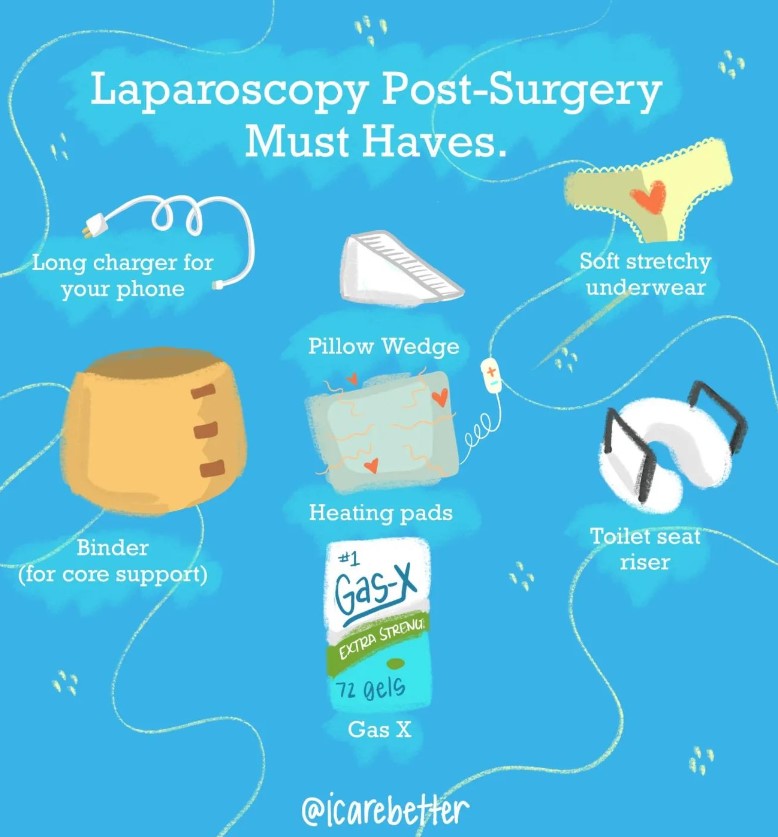
| Laparoscopy | A surgical procedure involving small incisions and the use of a camera to diagnose or treat conditions. | /lap·uh·ros·kuh·pee/ |
| Laparotomy | A surgical procedure involving a large incision through the abdominal wall to gain access to the abdominal cavity. | /lap·uh·rot·uh·mee/ |
| Laparoscopic Excision | A minimally invasive surgical technique used to remove endometriosis lesions. | /lap·uh·roh·skop·ik ek·si·zhun/ |
| Lupron | A medication used to treat endometriosis by suppressing estrogen production. | /loo·pron/ |
| Menarche | The first occurrence of menstruation. | /men·ahr·kee/ |
| Menopause | The time in a woman’s life when menstrual periods permanently stop. | /men·uh·pawz/ |
| Menorrhagia | Heavy menstrual bleeding. | /men·uh·ray·jee·uh/ |
| Myometrium | The muscular layer of the uterine wall. | /my·oh·mee·tree·um/ |
| Neurectomy | Surgical removal of a nerve or part of a nerve. | /noo·rek·tuh·mee/ |
| Oophorectomy | Surgical removal of one or both ovaries. | /oh·uh·fuh·rek·tuh·mee/ |
| Oral Contraceptives | Birth control pills that contain hormones to prevent pregnancy. | /awr·uhl kon·truh·sep·tivz/ |
| Ovarian Cyst | A fluid-filled sac within the ovary. | /oh·vair·ee·uhn sist/ |
| Ovary | The female reproductive organ that produces eggs and hormones. | /oh·vuh·ree/ |
| Pelvic Floor Dysfunction | A condition where the muscles and tissues supporting the pelvic organs are weakened. | /pel·vik flawr dis·funk·shun/ |
| Pelvic Inflammatory Disease (PID) | Infection of the female reproductive organs. | /pel·vik in·flam·uh·tor·ee dih·zeez/ |
| Peritoneum | The membrane lining the abdominal cavity and covering the abdominal and pelvic organs. | /per·i·toh·nee·um/ |
| Progesterone | A hormone involved in the menstrual cycle, pregnancy, and embryogenesis. | /proh·jes·tuh·rohn/ |
| Progestins | Synthetic hormones similar to progesterone. | /proh·jes·tinz/ |
| Rectovaginal Septum | The tissue between the rectum and the vagina. | /rek·toh·vaj·in·uhl sep·tuhm/ |
| Reproductive Endocrinologist | A doctor who specializes in reproductive hormones and fertility issues. | /ree·proh·duk·tiv en·doh·kri·nah·luh·jist/ |
| Resection | Surgical removal of part of an organ or structure. | /ri·sek·shun/ |
| Retrograde Menstruation | The backward flow of menstrual blood into the pelvic cavity. | /re·troh·grayd men·stroo·ay·shun/ |
| Retroverted Uterus | A uterus that tilts backward instead of forward. | /reh·troh·vur·tid yoo·tuh·rus/ |
Robotic Surgery Salpingectomy | Minimally invasive laparoscopic surgery enhanced by robotic tech Surgical removal of one or both fallopian tubes. | /roh·bot·tick sir·jury/ |
| Sonohysterography | An ultrasound procedure to examine the inside of the uterus. | /soh·noh·his·ter·oh·grah·fee/ |
| Subfertility | Reduced level of fertility characterized by unusually long time to conceive. | /sub·fur·til·i·tee/ |
| Transabdominal Ultrasound | An ultrasound test performed through the abdomen. | /tranz·ab·doh·mi·nuhl ul·truh·sownd/ |
| Transcervical | Through the cervix. | /tranz·sur·vik·uhl/ |
| Transvaginal Ultrasound | An imaging test using sound waves to look at the reproductive organs. | /tranz·vuh·jy·nuhl ul·truh·sownd/ |
| Ultrasound | An imaging method that uses high-frequency sound waves to capture live images from inside the body. | /uhl·truh·sownd/ |
| Uterine Fibroids | Noncancerous growths in the uterus. | /yoo·ter·in fye·broidz/ |
| Uterus | The organ in the female reproductive system where a fetus develops. | /yoo·tuh·rus/ |
| Vaginal Atrophy | Thinning, drying, and inflammation of the vaginal walls due to decreased estrogen. | /vaj·in·uhl at·ruh·fee/ |
| Vaginismus | Involuntary muscle spasms in the pelvic floor muscles. | /vaj·in·iz·muhs/ |
| Vulvodynia | Chronic pain or discomfort around the opening of the vagina. | /vuhl·voh·din·ee·uh/ |
Updated Post: July 16, 2024

Endometriosis Fertility Treatment: Natural, Medical, & Surgical Options
Table of contents
Natural, Medical & Surgical Treatment of Endometriosis Infertility
Endometriosis (endo) is a common condition that affects up to 10% of all women globally. But most people do not realize this condition’s impact on a significant proportion of women. Endometriosis and pregnancy complications are a common coincidence. Up to 50% of women with infertility have endo.
Endometriosis and pregnancy can be problematic for patients. And sometimes, endometriosis treatments are needed to conceive. Keep reading to learn more about fertility options for women with endo.
Read More: How Does Endometriosis Cause Infertility?
Lack of Evidence-Based Research Stalls Treatment Options
Endometriosis is sometimes like the elephant in the room that no one wants to discuss or do enough research. However, that needs to change because endometriosis is often a disabling condition, and people should know about it. Not only does this condition impact the patient’s quality of life, but it also affects the potential for some of these patients to have a family. This situation can affect a marriage, other family members, a partner, etc.
Options for treating women with endometriosis and pregnancy issues can be natural, medical, surgical, or surgery-assisted. Let’s review the latest fertility treatments and courses of action for women affected by endometriosis. First, we will briefly discuss how endometriosis and pregnancy are related.
Can You Get Pregnant with Endometriosis?
Natural
Getting pregnant with endometriosis is not always easy, but it’s a reality for most patients who have the condition and want to conceive. It’s important to emphasize that the body can and still does get pregnant. There are things such as an endometriosis diet that might help. Let’s look at the good numbers. Up to 70%, according to some studies, of women with mild to moderate endometriosis will become pregnant without medical intervention.
Medically-Assisted
Statistics show that about 75% of women with severe endometriosis (stage III/IV) will conceive if they desire. Two-thirds of those pregnancies occurred naturally, and one-third with the help of the endometriosis fertility treatment.
If you have endometriosis and are having troubles getting or maintaining a pregnancy, and you wish to carry full-term, here are some medical options that may interest you:
- Freeze some eggs: Your ovarian reserve of eggs can decline due to endometriosis. Therefore, some endo specialists recommend preserving your eggs in case you wish to conceive later. Just note that this can be an expensive option.
- Superovulation and intrauterine insemination (SO-IUI): If you have normal fallopian tubes, mild endometriosis, and a partner with healthy sperm, this might be the best choice for you.
- Fertility medications: Doctors can prescribe medications to produce up to two or three mature eggs. There are also progestin injections that are often used to help fertility issues.
- Frequent ultrasounds: If a person is trying to get pregnant, they may go in for frequent ultrasounds to identify when the eggs are most mature. At that time, a doctor can insert the partner’s collected sperm.
- In-Vitro Fertilization (IVF): This treatment involves the extraction of the egg and sperm. The egg is fertilized outside the body and then implanted into the uterus.
Endometriosis Surgery For Infertility
Many women with endometriosis do become pregnant without medical assistance. However, studies suggest that endometriosis surgery does help a woman to become pregnant without difficulty.
- Removal of endometriosis tissue: Evidence shows that pregnancy rates improve if the endometriosis tissues are removed surgically.
- Removal of tissue or large endometriosis cysts: Large cysts and tissue accumulation can contribute to infertility. Removing these can help the patient conceive.
- Routine follow-up: Women with endometriosis often have cysts that relapse after treatment. It is crucial to complete follow-up visits and possibly have complementary surgeries down the road.
How Your Stage of Endometriosis Impacts Fertility
A diagnosis of endometriosis is a heavy thing to take in, primarily since it’s known to impact a woman’s reproductive organs. Studies have shown that the extent of endometriosis present during laparoscopy correlates with fertility.
Do You Have Concerns About Endometriosis and Fertility?
We want to hear from you. What is your biggest concern about the fertility impact of endometriosis? Or does it concern you at all? Leave your answers in the comments below. If you need medical attention that is not emergent, be sure to find a vetted endometriosis specialist who is familiar with the disease and modern treatments.
Leptin and Its Role in Endometriosis
We’ve heard about leptin and its role in weight gain/loss, but did you know leptin does more than that?
“Subsequent work has confirmed that leptin has a pleiotrophic role on the immune response and can rightly be considered, both structurally and functionally, as a proinflammatory cytokine (Lord, 2006, p. 151).” (Note: Pleiotrophic means one gene that influence many, so leptin can influence lots of different things.) Also, in a very small study, leptin was tied to greater fatigue in CFS patients: “Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology (Stringer et al., 2013, p.1).
So, leptin can be proinflammatory and influence many different responses in the body (including energy/fatigue and immune response). The following studies demonstrate that leptin (similar to the effects of estrogen) can cause endometriosis lesions to proliferate (to get bigger and bulkier).
This study explores leptin’s role in endometriosis and its effect on inflammation and angiogenesis (creating new blood vessels). It also found that leptin had a different effect on cells in the uterus versus endometriosis lesions (highlighting that endometriosis cells have distinct differences from the lining of the uterus).
- Wu, M. H., Chuang, P. C., Chen, H. M., Lin, C. C., & Tsai, S. J. (2002). Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Molecular human reproduction, 8(5), 456-464. Retrieved from https://academic.oup.com/molehr/article/8/5/456/1030708
“Leptin has been reported to exert immunoregulatory, proinflammatory, mitogenic and angiogenic effects in several tissues (Gainsford et al., 1996; Wolf et al., 1999; Caprio et al., 2001). This makes it a potential candidate for contributing to the progress of endometriosis. A recent report even demonstrated that leptin levels in peritoneal fluid and serum of patients with pelvic endometriosis are increased (Matarese et al., 2000). However, the cellular origin and mechanism by which leptin modulates the formation of endometriosis is not clear. We herein present evidence showing that leptin and its receptor are differentially expressed in endometriosis and are involved in the proliferation of endometrial stromal cells….
“…leptin stimulated a significant increase in eutopic as well as ectopic endometrial stromal cell proliferation. However, this mitogenic effect of leptin was somewhat different in eutopic endometrial stromal cells compared with ectopic endometriotic stromal cells. In eutopic endometrial stromal cells, leptin caused a greater extent of cell proliferation and at much lower doses (Figure 8A). In stromal cells obtained from ectopic endometriotic implants, only high doses of leptin (3 and 10 ng/ml) induced cell proliferation and the induction was less pronounced (Figure 8B)….
“…we showed that both leptin transcripts and protein are highly expressed in ectopic endometriotic lesions. In eutopic endometrium, leptin was not detected in a half of the samples and only extremely low amounts of leptin were detected in the other half of the endometria. In concordance with our finding, contradictory reports have shown either positive or negative leptin expression in normal human endometrium (Alfer et al., 2000; Gonzalez et al., 2000a; Kitawaki et al., 2000). The reasons for differences in leptin transcript expression in eutopic endometrium are not known. Nevertheless, leptin was highly expressed in ectopic endometriotic lesions. The elevation of leptin in ectopic endometriosis was not due to differences in the stages of menstrual cycles or body mass as evidenced by marked increase of leptin in ectopic endometriotic tissues as compared to the eutopic endometrium collected from the same patients (n = 4). In addition, the mean BMI was not different between eutopic and endometriosis groups. Thus, elevated expression of leptin in ectopic endometriotic tissues may reflect the distinct biochemical nature of endometriotic lesions. Our result showing that leptin is markedly expressed in ectopic endometriotic lesions supports previous reports that the peritoneal fluid concentration of leptin was increased in women with endometriosis (Matarese et al., 2000; De Placido et al., 2001)….One of the examples is the acquisition of estrogen-producing ability in ectopic endometriotic implants (Noble et al., 1996; Bulun et al., 1999, 2000). Our recent data have also indicated that ectopic endometriotic cells of early endometriosis express high quantities of steroidogenic acute regulatory protein and produce high levels of progesterone (Tsai et al., 2001c). As a consequence, the ectopic endometriotic tissues become independent of the survival factors generated from gonads, and proliferate continuously throughout the cycle….In summary, differential expression of leptin and its receptor in eutopic and ectopic endometrium suggests that leptin may have a critical role in endometriosis development. Elevated leptin expression by endometriosis lesions appears to enhance the proliferation of ectopic endometriotic stromal cells. Our findings may open a new field of investigation into the actions of leptin in the pathogenesis of endometriosis and provide a reasonable rationale for developing a therapeutic regime for endometriosis by targeting leptin and its action.”
In the following study, the authors further investigate the role of leptin in endometriosis through its inflammatory and angiogenic properties:
- Nácul, A. P., Lecke, S. B., Edelweiss, M. I., Morsch, D. M., & Spritzer, P. M. (2013). Gene expression of leptin and long leptin receptor isoform in endometriosis: a case-control study. Obstetrics and gynecology international, 2013. Retrieved from http://www.hindawi.com/journals/ogi/2013/879618/
“It (Leptin) may also play a role in endometriosis through its inflammatory and angiogenic properties…. Moreover, the possibility of an association between PF leptin levels and severity of endometriosis is also controversial, with some studies suggesting a negative correlation [2, 6, 8] and others showing a positive correlation with more severe forms of peritoneal endometriosis [5, 7, 13, 15]….Conclusions: The present data suggest that serum leptin/BMI ratio is associated with the presence of endometriosis. Nevertheless, the clinical applicability of the leptin/BMI ratio for prediction of endometriosis still requires confirmation. Moreover,the increased expression of leptin and OB-RL in ectopic endometrium suggests a modulatory interaction between leptin and its active receptor and a role of leptin, an inflammatory and angiogenic cytokine, in the initiation or development of endometrial implants.”
In this study, the authors elaborate on the increased leptin expression in endometriosis cells causing them to proliferate:
- Wu, M. H., Chuang, P. C., Chen, H. M., Lin, C. C., & Tsai, S. J. (2002). Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Molecular human reproduction, 8(5), 456-464. Retrieved from https://pubmed.ncbi.nlm.nih.gov/11994543/
“Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Abstract: Endometriosis is a polygenic disease with complex, multifactorial aetiologies affecting approximately 10% of women of reproductive age. Leptin is the product of the ob gene, which is related to reproductive function and immunological alteration. The angiogenic and mitogenic action of leptin may influence the formation of endometriosis. This study was aimed at determining whether leptin and leptin receptor expression differs in eutopic and ectopic endometria collected from laparoscopy and at investigating the pathophysiological role of leptin in the development of endometriosis. Leptin mRNA was undetectable in seven out of 14 eutopic endometria and only a minute amount was detected in the remaining samples. In contrast, there was a marked increase in leptin mRNA and protein expression in ectopic endometriotic lesions of patients with endometriosis (P < 0.05). Receptors for leptin were immunologically stained in eutopic endometrium as well as in ectopic endometriotic implants. However, the levels of mRNA for the long and total forms of leptin receptors were suppressed in association with the severity of endometriosis (P < 0.05). Administration of leptin stimulated its own mRNA expression in ectopic endometriotic stromal cells but decreased steady-state concentrations of mRNA encoding for leptin receptor (n = 6). In addition, leptin significantly enhanced both eutopic and ectopic endometrial stromal cell proliferation (P < 0.05). In conclusion, the differential distribution of mRNA for leptin and its receptor suggests an important autocrine and paracrine role for leptin in human endometriosis. The mitogenic and auto-augmentation effects of leptin may further contribute to the pathogenesis of endometriosis.”
The following study discusses leptin stimulating the increased growth of endometriosis cells:
- Oh, H. K., Choi, Y. S., Yang, Y. I., Kim, J. H., Leung, P. C., & Choi, J. H. (2012). Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. MHR: Basic science of reproductive medicine, 19(3), 160-168. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23184927
“Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Leptin acts as a potential growth stimulator in several normal and neoplastic cells. Recent studies have shown the presence of increased levels of leptin in the peritoneal fluid of patients with endometriosis, implicating leptin in the pathogenesis of endometriosis. However, the specific function of leptin in the induction of mitogenesis in endometriosis is not known. This study investigated the expression of the leptin receptor (ObR) in endometrioma tissues and immortalized endometriotic cells, and the effect of leptin on cell growth. ObR expression was higher in endometriomas than in the normal endometrium, and it was detected in 74% of epithelial and 30% of stromal endometrioma tissues. In addition, human endometriotic epithelial cells (11Z and 12Z) showed a high level of ObR when compared with endometrial cells and endometriotic stromal cells (22B). Furthermore, leptin treatment stimulated the growth of 11Z and 12Z cells, but not that of 22B cells. Knockdown of the ObR in 11Z and 12Z cells impaired the ability of leptin to induce cell growth. Leptin induced the activation of Janus Kinases 2 (JAK2), signal transducers and activators of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) in endometriotic epithelial cells. Moreover, pretreatment with the JAK2/STAT3 inhibitor AG490 and the ERK inhibitor PD98059 significantly inhibited leptin-induced cell growth. The present results show that the ObR is induced in endometriosis, and that leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways.”
References
Lord, G. M. (2006). Leptin as a proinflammatory cytokine. In Obesity and the Kidney (Vol. 151, pp. 151-164). Karger Publishers. Retrieved from https://pubmed.ncbi.nlm.nih.gov/16929139/
Stringer, E. A., Baker, K. S., Carroll, I. R., Montoya, J. G., Chu, L., Maecker, H. T., & Younger, J. W. (2013). Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. Journal of translational medicine, 11(1), 93. Retrieved from http://www.translational-medicine.com/content/11/1/93
Progesterone Resistance in Endometriosis
The following studies elaborate on progesterone resistance in endometriosis lesions. This progesterone resistance could provide a clue as to why some people do not respond to progestin therapy. It also is a clue as to why estrogen exerts such a strong role on endometriosis- because there is not a balancing progesterone effect.
- Cheng, Y. H., Imir, A., Fenkci, V., Yilmaz, M. B., & Bulun, S. E. (2007). Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17β-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. American journal of obstetrics and gynecology, 196(4), 391-e1. Retrieved from http://www.ncbi.nlm.nih.gov/m/pubmed/17403431/?i=5&from=/9851796/related
“CONCLUSION: A stromal cell defect in endometriosis blocks formation of progesterone-dependent production of factors leading to 17beta-hydroxysteroid dehydrogenase type 2 deficiency and defective conversion of estradiol to estrone in epithelium.”
- Bruner-Tran, K. L., Herington, J. L., Duleba, A. J., Taylor, H. S., & Osteen, K. G. (2013). Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva ginecologica, 65(2), 199. Retrieved from http://europepmc.org/articles/PMC3718308
“…initial studies comparing endometrial tissues from women with and without endometriosis examined circulating progesterone levels relative to expected histological responses across the secretory phase of the menstrual cycle 27–29. These investigations revealed that while women with endometriosis exhibit normal circulating ovarian progesterone levels, the endometrium’s ability to respond appropriately to this steroid appeared to be reduced 27–29. Subsequent studies confirmed that endometrial tissues from women with endometriosis did not exhibit the changes in specific gene and protein expression normally expected during the progesterone-dominated secretory phase 12, 30–31. Perhaps not surprisingly, altered expression of genes and proteins in endometriosis patients was reported to be associated with changes in the expression pattern of progesterone receptor (PR) isotypes (PR-A and PR-B), at both eutopic and ectopic sites of endometrial growth11, 32–33….At present, the biological origin of reduced endometrial progesterone responsiveness among women with endometriosis remains to be fully elucidated; however, a number of research groups have begun to examine whether chronic inflammatory processes may promote the development of endometrial resistance to this steroid. Within the reproductive tract, an important component of steroidal regulation of inflammation involves cellular signaling by members of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) family. This signaling network has been suggested to play a critical role in triggering, or enhancing, the inflammatory processes…Although the studies noted above support the concept that inflammatory processes may represent a potential trigger for the loss of progesterone sensitivity related to endometriosis, the precise cellular and molecular mechanisms leading to this disease phenotype remain elusive. In this regard, a number of recent observations suggest that epigenetic modification, mediated by chronic inflammation, could explain the progesterone resistant endometrial phenotype observed in women with endometriosis. ”
- Al-Sabbagh, M., Lam, E. W. F., & Brosens, J. J. (2012). Mechanisms of endometrial progesterone resistance. Molecular and cellular endocrinology, 358(2), 208-215. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22085558
“Throughout the reproductive years, the rise and fall in ovarian hormones elicit in the endometrium waves of cell proliferation, differentiation, recruitment of inflammatory cells, apoptosis, tissue breakdown and regeneration. The activated progesterone receptor, a member of the superfamily of ligand-dependent transcription factors, is the master regulator of this intense tissue remodelling process in the uterus. Its activity is tightly regulated by interaction with cell-specific transcription factors and coregulators as well as by specific posttranslational modifications that respond dynamically to a variety of environmental and inflammatory signals. Endometriosis, a chronic inflammatory disorder, disrupts coordinated progesterone responses throughout the reproductive tract, including in the endometrium. This phenomenon is increasingly referred to as ‘progesterone resistance’. Emerging evidence suggests that progesterone resistance in endometriosis is not just a consequence of perturbed progesterone signal transduction caused by chronic inflammation but associated with epigenetic chromatin changes that determine the intrinsic responsiveness of endometrial cells to differentiation cues.”
Why is this progesterone resistance in endometriosis lesions important? Because progesterone helps in the process of metabolizing estrogen:
- Bulun, S. E., Cheng, Y. H., Yin, P., Imir, G., Utsunomiya, H., Attar, E., … & Kim, J. J. (2006). Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Molecular and cellular endocrinology, 248(1-2), 94-103. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16406281
“The biologically active estrogen estradiol (E2) is the best-defined mitogen for the growth and inflammation processes in the ectopic endometriotic tissue that commonly resides on the pelvic organs. Progesterone and progestins may relieve pain by limiting growth and inflammation in endometriosis but a portion of patients with endometriosis and pelvic pain do not respond to treatment with progestins. Moreover, progesterone-induced molecular changes in the eutopic (intrauterine) endometrial tissue of women with endometriosis are either blunted or undetectable. The molecular basis of progesterone resistance in endometriosis may be related to an overall reduction in the levels of progesterone receptors (PRs) and the lack of the PR isoform named progesterone receptor B (PR-B). In normal endometrium, progesterone acts on stromal cells to induce secretion of paracrine factor(s). These unknown factor(s) act on neighboring epithelial cells to induce the expression of the enzyme 17beta-hydroxysteroid dehydrogenase type 2 (17beta-HSD-2), which metabolizes the biologically active estrogen E2 to estrone (E1). In endometriotic tissue, progesterone does not induce epithelial 17beta-HSD-2 expression due to a defect in stromal cells. The inability of endometriotic stromal cells to produce progesterone-induced paracrine factors that stimulate 17beta-HSD-2 may be due to the lack of PR-B and very low levels of progesterone receptor A (PR-A) observed in vivo in endometriotic tissue. The end result is deficient metabolism of E2 in endometriosis giving rise to high local concentrations of this local mitogen.”
- Zeitoun, K., Takayama, K., Sasano, H., Suzuki, T., Moghrabi, N., Andersson, S., … & Bulun, S. E. (1998). Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. The Journal of Clinical Endocrinology & Metabolism, 83(12), 4474-4480. Retrieved from http://www.ncbi.nlm.nih.gov/m/pubmed/9851796/?i=3&from=/16406281/related
“In conclusion, inactivation of 17beta-estradiol is impaired in endometriotic tissues due to deficient expression of 17betaHSD-2, which is normally expressed in eutopic endometrium in response to progesterone. The lack of 17betaHSD-2 expression in endometriosis is not due to alterations in the levels of immunoreactive progesterone or estrogen receptors in this tissue and may be related to an inhibitory aberration in the signaling pathway that regulates 17betaHSD-2 expression.”
- Matsuzaki, S., Canis, M., Pouly, J. L., Déchelotte, P. J., & Mage, G. (2006). Analysis of aromatase and 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression in deep endometriosis and eutopic endometrium using laser capture microdissection. Fertility and sterility, 85(2), 308-313. Retrieved from http://www.ncbi.nlm.nih.gov/m/pubmed/16595205/?i=2&from=/9851796/related
“CONCLUSION(S): Local estrogen concentration may be much higher in epithelial cells than in stromal cells in deep endometriotic tissue.”
Furthermore (part of this is particular for those with infertility):
- Sirota, I., Zarek, S. M., & Segars, J. H. (2014, January). Potential influence of the microbiome on infertility and assisted reproductive technology. In Seminars in reproductive medicine (Vol. 32, No. 01, pp. 035-042). Thieme Medical Publishers. Retrieved from https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0033-1361821
“On the basis of the role of inflammation in the induction of a progesterone-resistant endometrium, we hypothesize that failure of implantation might be explained, perhaps in part, by alteration in the uterine microbiome in response to inflammation…This hypothesis finds support in the ability of environmental factors to alter progesterone sensitivity…A very important finding derived from this study demonstrated that TCDD-mediated P4 resistance might increase sensitivity to inflammation, even in subsequent generations not exposed to the toxicants, resulting in PTB.[98] By means of extrapolation, these data support the hypothesis that a combination of environmental factors, taken together, are a risk for recurrent pregnancy loss and PTB in humans; the mechanism being creation of a P4-resistant endometrium and the presence of inflammation. Therefore, we conclude that the association between the microbiome of the reproductive tract and circulating serum E2 concentrations may reflect the environment and availability of glycogen. However, progesterone resistance, albeit an unproven relationship to the microbiome, might contribute to implantation failure and infertility. This putative role of undetected endometrial colonization and progesterone resistance requires further investigation.”
- Houshdaran, S., Oke, A. B., Fung, J. C., Vo, K. C., Nezhat, C., & Giudice, L. C. (2020). Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS genetics, 16(6), e1008601. https://doi.org/10.1371/journal.pgen.1008601
“The cells were derived from normal woman and those with endometriosis, an E2-responsive, P4-resistant, inflammatory disorder with implantation-based infertility and poor pregnancy outcomes.”
Weird places endometriosis has been found
Endometriosis Where?!?
Although very rare, endometriosis can show up in surprising places. Here are a few case studies:
Vaginal Cuff
- Arkerson, B. J., Wyckoff, E. T., & Moawad, N. S. (2018). Vaginal Cuff Endometriosis with Endometrial Hyperplasia: A Rare Cause of Post-Hysterectomy Vaginal Bleeding. Journal of Gynecologic Surgery, 34(2), 92-94. https://www.liebertpub.com/doi/abs/10.1089/gyn.2017.0075
“A 53-year-old female presented with postmenopausal bleeding. She had undergone a total TAH/BSO in 2001 for menorrhagia and uterine fibroids. The operative report described an uncomplicated procedure, and pathology was remarkable for inactive endometrium, adenomyosis, small fibroids, and normal ovaries, without evidence of endometriosis. Since then, the patient had used oral estrogen replacement. On presentation, ultrasonography showed no pelvic masses or fluid collections. She did have vaginal cuff granulation tissue and tenderness on bimanual examination. A vaginal cuff biopsy revealed endometriosis with simple hyperplasia without atypia. The patient elected to have laparoscopic vaginal cuff revision with removal of the vaginal cuff endometriosis that was demarcated by injectable dye as a guide. Results: The patient’s postoperative recovery was uneventful. No bleeding or pain was noted during a 2-year follow-up period. She was not restarted on estrogen replacement to minimize the risk of recurrence. Conclusions: Laparoscopic vaginal cuff revision with the use of injectable dye to ensure complete excision of cuff endometriosis is a feasible and safe method for the management of symptomatic vaginal cuff lesions following hysterectomy.”
Inguinal Hernia
- Yu, M., & McKay, G. (2018, September). Inguinal Endometriosis; Diagnostic Considerations & Management. In AUSTRALIAN & NEW ZEALAND JOURNAL OF OBSTETRICS & GYNAECOLOGY (Vol. 58, pp. 88-88). 111 RIVER ST, HOBOKEN 07030-5774, NJ USA: WILEY. Retrieved from https://www.ranzcogasm.com.au/wp-content/uploads/2018/09/Yu-Michelle-193.pdf
“A 20 year old nulliparous female presented with a right sided inguinal lump, her pain and swelling worse during menstruation. She had no other previous surgical or medical history. The mass was non-reducible with features of a femoral hernia. Ultrasound reported a lobulated hypoechoic fluid filled structure within the inguinal canal above the superficial ring. Further imaging modalities were considered but not deemed suitable in this particular case with consideration of future fertility. Based on clinical findings suspecting groin hernia, the patient underwent laparoscopic mesh repair of hernia. Surgery confirmed a right side indirect inguinal hernia arising from a patent indirect sac. The sac was reduced with traction and on routine transection chocolate brown fluid escaped. Mesh was placed in the pre-peritoneal plane over the defect to complete the hernorrhaphy. Histopathology of sac and cystic contents showed an inguinal hernia sac with endometriosis. Her postoperative recovery was unremarkable.”
Lungs (Pulmonary):
- Foster, D. C., Stern, J. L., Buscema, J., Rock, J. A., & Woodruff, J. D. (1981). Pleural and parenchymal pulmonary endometriosis. Obstetrics & Gynecology, 58(5), 552-556. Retrieved from https://journals.lww.com/greenjournal/abstract/1981/11000/pleural_and_parenchymal_pulmonary_endometriosis.3.aspx
“Sixty-five cases of pulmonary endometriosis are reviewed for characteristics of age, parity, prior surgery, prior endometriosis, location of pulmonary disease, and documentation of disease. Two categories of pulmonary endometriosis are defined: 1) pleural and 2) parenchymal. Age, history of pelvic endometriosis, and location of disease were found to be significantly different between the 2 groups.”
Liver (Hepatic):
- Roesch-Dietlen, F., Jiménez-García, A., Pérez-Morales, A., Grube-Pagola, P., Ramírez-Cervantes, K. L., & Remes-Troche, J. M. (2016). Hepatic endometriosis. Annals of hepatology, 10(3), 347-348. Retrieved from https://www.medigraphic.com/pdfs/hepato/ah-2011/ah113n.pdf
“Here, we present a case of an incidental intraparenchymal hepatic endometriosis in a young woman who presented with only right upper quadrant pain. A 25-year-old nulliparous woman was referred to our unit due to an 8-month history of relapsing and remitting right upper quadrant pain. In the last month, these episodes occurred more often and were related to the ingestion of fatty foods. There were no other symptoms.”
Skin (Cutaneous):
- Steck, W. D., & Helwig, E. B. (1965). Cutaneous endometriosis. JAMA, 191(3), 167-170. Retrieved from https://jamanetwork.com/journals/jama/article-abstract/654568
“A clinicopathologic study of cutaneous endometriosis in 82 patients includes 28 lesions of the umbilicus, 42 of the lower abdominal wall, and 12 in the inguinal area, labia, and perineum. With the exception of five endometriomas of the inguinal area, every lesion occurred in a scar. Twenty-one arose without a preceding operation in the physiologic scar of the umbilicus, and 56 in surgical scars. The umbilical lesions were too precisely located to be logically explained by lymphatic spread, unless only the cells that find scar tissue are able to proliferate. Cesarean-section scars of 26 patients were involved, indicating a vastly greater incidence of endometriosis in cesarean scars than previously reported. This either casts doubt on the theory of transplantation as the usual pathogenetic mechanism for the lesions in surgical scars, or suggests that the endometrium of pregnancy is easier to transplant than is commonly believed.”
Sciatic Nerve:
- Lomoro, P., Simonetti, I., Nanni, A., Cassone, R., Di Pietto, F., Vinci, G., … & Sammarchi, L. (2019). Extrapelvic Sciatic Nerve Endometriosis, the Role of Magnetic Resonance Imaging: Case Report and Systematic Review. Journal of Computer Assisted Tomography, 43(6), 976-980. Retrieved from https://journals.lww.com/jcat/Abstract/2019/11000/Extrapelvic_Sciatic_Nerve_Endometriosis,_the_Role.25.aspx
“Endometriosis (EN) is a common gynecological condition characterized by the presence of functional endometrium located outside the uterine cavity. Sciatic nerve (SN) is rarely affected by EN. Magnetic resonance imaging allows a direct visualization of the spinal and SN, and it is the modality of choice for the study of SN involvement in extrapelvic EN. We report a case of an endometrioma located in the right SN with a systematic review of the literature.”
- Roca, M. U., Bandeo, L., Saucedo, M. A., Bala, M., Binaghi, D., Chertcoff, A., … & Pardal, M. F. (2019). Cyclic Sciatica: Presentation of a Case With Intra and Extrapelvic Endometriosis Affecting the Sciatic Nerve and Utility of MR Neurography (P3. 4-026). Retrieved from https://n.neurology.org/content/92/15_Supplement/P3.4-026.abstract
“A 35-year-old female patient consulted for right low back pain extending along her posterior thigh, calf and foot since 2 years. The pain was recurrent, acute in onset, lasted several days and gradually diminished until disappearing. It was refractory to common analgesics and during the crisis she had difficulties to walk. Neurologist requested a calendar of pain in which the relationship between the menstrual cycle and the pain became evidenced. We performed MRN of the lumbo sacral plexus that showed multiple endometriotic implants in ovaries, L5-S1 roots and a huge one on the sciatic nerve (intra and extrapelvic segment). The patient started oral contraceptives but presented progressive worsening of pain until it became constant and developed step page. Electromyogram showed acute and chronic axonal damage in the sciatic nerve distribution. Medical treatment was changed to leuprolide acetate. The patient evolved with improvement of ovarian endometriosis but persistence of sciatic nerve lesions, leg pain and weakness up to now. Surgical option was considered.”
- Yanchun, L., Yunhe, Z., Meng, X., Shuqin, C., Qingtang, Z., & Shuzhong, Y. (2019). Removal of an endometrioma passing through the left greater sciatic foramen using a concomitant laparoscopic and transgluteal approach: case report. BMC women’s health, 19(1), 95. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6624926/
“A 20-year-old woman presented with complaints of severe dysmenorrhea lasting for more than 6 years and dysfunction of her left lower limb lasting for approximately 4 months. Both CT and MRI demonstrated a suspected intrapelvic and extrapelvic endometriotic cyst (7.3 cm × 8.1 cm × 6.5 cm) passing through the left greater sciatic foramen. Laparoscopic exploration showed a cyst full of dark fluid occupying the left obturator fossa and extending outside the pelvis. A novel combination of transgluteal laparoscopy was performed for complete resection of the cyst and decompression of the sciatic nerve. Postoperative pathology confirmed the diagnosis of endometriosis. Long-term follow-up observation showed persistent pain relief and lower limb function recovery in the patient.”
Brain
- Meggyesy, M., Friese, M., Gottschalk, J., & Kehler, U. (2020). Case Report of Cerebellar Endometriosis. Journal of Neurological Surgery Part A: Central European Neurosurgery. Retrieved from https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0040-1701622
“We describe a case of cerebellar endometriosis in a 39-year-old woman who underwent posterior fossa decompression multiple times without establishing a correct diagnosis. Her neurologic status progressively worsened due to chronic hydrocephalus and brainstem compression by cysts. Late in the clinical course, histology from the cyst wall was taken that revealed endometriosis with clear cells and positive immunohistology for progesterone and estrogen receptors. Treatment with gestagens was started but did not improve the patient’s status. In patients with chronic recurring intracranial cysts and hydrocephalus, cerebral endometriosis should be considered.”
Heart (Cardiac):
- Sarmast, H., & Takriti, S. Z. (2019). Cardiac Involvement Resulting from Thoracic Endometriosis. Cardiovasc Surg Int, 1(1), 1002. Retrieved from https://www.researchgate.net/profile/Hossein_Sarmast2/publication/338832751_Cardiac_involvement_resulting_from_thoracic_endometriosis/links/5e2ea4ad299bf1e929d93270/Cardiac-involvement-resulting-from-thoracic-endometriosis.pdf
“We reported a 28-years old woman who suffered from thoracic endometriosis syndrome accompanied by cardiac involvement. Also our patient is the third report of surgically documented thoracic endometriosis syndrome, involving right side pleura and pericardium.”
- Video of pericardial endometriosis surgery
- Video of pericardial and diaphragmatic endometriosis surgery
Locations of endometriosis
Studies:
- Charatsi, D., Koukoura, O., Ntavela, I. G., Chintziou, F., Gkorila, G., Tsagkoulis, M., … & Daponte, A. (2018). Gastrointestinal and urinary tract endometriosis: a review on the commonest locations of extrapelvic endometriosis. Advances in medicine, 2018. Retrieved from https://www.hindawi.com/journals/amed/2018/3461209/
“Endometriotic lesions have been reported in every part of the female human body and in some instances in males. Organs that are close to the uterus are more often affected than distant locations. Extrapelvic endometriosis affects a slightly older population of women than pelvic endometriosis….The gastrointestinal tract is the most common location of extrapelvic endometriosis with the urinary system being the second one. However, since sigmoid colon, rectum, and bladder are pelvic organs, extragenital pelvic endometriosis may be a more suitable definition for endometriotic implants related to these organs than extrapelvic endometriosis. The sigmoid colon is the most commonly involved, followed by the rectum, ileum, appendix, and caecum. Most lesions are confined in the serosal layer; however, deeper lesion can alter bowel function and cause symptoms. Bladder and ureteral involvement are the most common sites concerning the urinary system. Unfortunately, ureteral endometriosis is often asymptomatic leading to silent obstructive uropathy and renal failure. Surgical excision of the endometriotic tissue is the ideal treatment for all types of extrapelvic endometriosis.
“Pelvic endometriosis usually refers to lesions proximal to the uterus such us the ovaries, the fallopian tubes, the uterine ligaments, and the surrounding pelvic peritoneum. Extrapelvic endometriosis on the other hand, is affecting other areas of the body, including the vagina, vulva, cervix and perineum, the urinary system, the gastrointestinal tract, the thoracic cavity including lung and pleura, extremities, skin, and central nervous system. Nevertheless, the term of extragenital pelvic endometriosis describes in a more accurate way endometriotic lesions involving pelvic organs such as rectum, sigmoid, and bladder.”
Histology of Endometriosis
Histological appearance of endometriosis refers to what it looks like under the microscope. This is done when a biopsy or removed tissue (excised tissue) is sent to the pathologist for confirmation.
Overall histological appearance:
- Busca, A. and Parra-Herran, C. (2017). Ovary: Other nonneoplastic: Endometriosis. PathologyOutlines.com. Retrieved from http://www.pathologyoutlines.com/topic/ovarynontumorendometriosis.html
“At least two of the following three microscopic features: Endometrial type glands, Endometrial type stroma, Evidence of chronic hemorrhage (hemosiderin laden macrophages)
- Glandular epithelium commonly has metaplastic changes (tubal, mucinous, squamous, hobnail)
- Atypical endometriosis: epithelial lining of the glands may show enlargement with abundant eosinophilic cytoplasm, cellular stratification and hyperchromatic nuclei; can be reactive but also has malignant potential and is considered the precursor lesion for endometriosis associated carcinomas (clear cell or endometrioid) (Histopathology 1997;30:249, Case Rep Oncol 2013;6:480)
- Burnt out endometriosis: this term has been proposed for changes suggestive of endometriosis, namely central necrosis with surrounding fibrosis and pseudoxanthoma cells but lacking confirmatory features as listed above
- Liesegang rings: acellular ring-like structures seen in areas of chronic inflammation”
Smooth muscle fibers in deep infiltrating endometriosis (DIE):
- Anaf, V., Simon, P., Fayt, I., & Noel, J. C. (2000). Smooth muscles are frequent components of endometriotic lesions. Human reproduction, 15(4), 767-771. Retrieved from https://academic.oup.com/humrep/article/15/4/767/706409
“Deep infiltrating endometriosis (deeper than 5 mm under the peritoneum) often takes the form of a nodular lesion (or `adenomyotic nodule’) consisting of smooth muscles and fibrosis with active glands and scanty stroma. Thus, among endometriotic lesions, a certain distinction is drawn between musculo-glandular lesions and glandular lesions composed of endometrial-like epithelium surrounded by a cell-producing (cytogenous) stroma. The aim of this study was to detect by immunohistochemistry, with a monoclonal antibody against muscle-specific actin, the presence of smooth muscles in 54 endometriotic lesions originating from four different pelvic locations (peritoneum, ovary, rectovaginal septum and uterosacral ligaments) and to quantify the smooth muscle content. Smooth muscles were frequent components of endometriotic lesions in pelvic locations. In addition, smooth muscles were significantly (P < 0.001) more abundant in endometriotic lesions than in their respective unaffected sites. This finding supports, at least partially, the occurrence of a metaplastic phenomenon in the pathogenesis of endometriotic lesions. The definition of distinct endometriotic entities based on the difference in the tissue composition of the lesions (endometriotic nodules versus adenomyotic nodules) is inconsistent with the very frequent presence of smooth muscle cells in endometriosis irrespective of its localization.”
The Many Appearances of Endo
Endometriosis has been described as appearing in many different colors: clear, white, red, yellow, brown, and black (Yeung, Sinervo, Winer, & Albee, 2011). Lesions have also been described as “petechial, vesicular, polypoid, hemorrhagic, red flame-like” (Agarwal & Subramanian, 2010). The appearance of endometriosis is important when surgery is performed to diagnose and treat endometriosis- otherwise it might be missed.
Links:
Studies:
- Yeung Jr, P., Sinervo, K., Winer, W., & Albee Jr, R. B. (2011). Complete laparoscopic excision of endometriosis in teenagers: is postoperative hormonal suppression necessary?. Fertility and sterility, 95(6), 1909-1912. Retrieved from https://www.fertstert.org/article/S0015-0282(11)00335-9/fulltext
“Endometriosis has many different appearances that can make the diagnosis challenging and may necessitate histologic confirmation. “Subtle” or “atypical” appearance has been described as “red” or “white” lesions, or “clear” vesicles. Endometriosis in teenagers has been found to be more atypical in appearance. Some believe that with enhanced magnification available with modern-day laparoscopy, virtually all endometriosis can be identified.”
- Agarwal, N., & Subramanian, A. (2010). Endometriosis–morphology, clinical presentations and molecular pathology. Journal of laboratory physicians, 2(1), 1. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3147077/
“…black, dark-brown, or bluish puckered lesions, nodules or small cysts containing old hemorrhage surrounded by a variable extent of fibrosis. Atypical or ‘subtle’ lesions are also common, including red implants (petechial, vesicular, polypoid, hemorrhagic, red flame-like) and serous or clear vesicles. Other appearances include white plaques or scarring and yellowish brown peritoneal discoloration of the peritoneum. Endometriomas usually contain thick fluid like tar; such cysts are often densely adherent to the peritoneum of the ovarian fossa and the surrounding fibrosis may involve the tubes and bowel. Deeply infiltrating endometriotic nodules extend >5 mm beneath the peritoneum and may involve the uterosacral ligaments, vagina, bowel, bladder, or ureters. The depth of infiltration is related to the type and severity of symptoms.”
- Demco, L. (2000). Review of pain associated with minimal endometriosis. JSLS: Journal of the Society of Laparoendoscopic Surgeons, 4(1), 5. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3015350/
“The classic black lesions were found to be painful in only 11% of patients when the lesion was touched. Similarly, white lesions were painful in 20% of patients with red lesions at 37%, and clear lesions at 32% were the most painful. These results added further reason as to why initial therapy had such poor results. Surgeons would only “see” the black lesions and removed them, but these were the least painful lesions. The most painful clear lesions were not “seen” at laparotomy and therefore remained, as did the pain.”
- Donnez, J., Squifflet, J., Casanas-Roux, F., Pirard, C., Jadoul, P., & Van, A. L. (2003). Typical and subtle atypical presentations of endometriosis. Obstetrics and gynecology clinics of North America, 30(1), 83-93. Retrieved from http://europepmc.org/article/med/12699259
“The diagnosis of peritoneal endometriosis at the time of laparoscopy is often made by the observation of typically puckered black or bluish lesions. There are also numerous subtle appearances of peritoneal endometriosis. The lesions are frequently non-pigmented. Red flame-like lesions, glandular excrescences, and subovarian adhesions must be considered as the most active lesions. Sometimes, however, subtle endometriotic lesions can be the only lesions seen at laparoscopy.”
- Farrugia, M., Nair, M. S., & Kotronis, K. V. (2007). Narrow band imaging in endometriosis. Journal of minimally invasive gynecology, 14(4), 393-394. Retrieved from https://www.jmig.org/article/S1553-4650(06)00663-7/abstract
“Endometriosis is a disease of protean appearances and, when present on the superficial peritoneum, may assume a range of coloration, from the obvious to the very subtle. The exact extent of a lesion may be difficult to judge because subtle lesions merge with normal peritoneum. An inflammatory reaction occurs in most, but not all superficial lesions. Endometriotic lesions are angiogenic and create an altered microvascular pattern on the peritoneal lining.”
- Albee Jr, R. B., Sinervo, K., & Fisher, D. T. (2008). Laparoscopic excision of lesions suggestive of endometriosis or otherwise atypical in appearance: relationship between visual findings and final histologic diagnosis. Journal of Minimally Invasive Gynecology, 15(1), 32-37. Retrieved from https://www.sciencedirect.com/science/article/pii/S1553465007010205
“The greatest number of patient lesions were excised from cul-de-sac (n = 309). For this site, using visual criteria for diagnosis of endometriosis, positive predictive value was 93.9%, sensitivity was 69.3%, negative predictive value was 41.9%, and specificity was 83.1%. Prevalence was noted to be 79.0% and accuracy was 72.2%. In addition, atypical-appearing tissue not presumed to be endometriosis was confirmed to be endometriosis histologically in 24.3%. In examining tissue specimens from multiple anatomic sites, laparoscopic visual diagnosis of typical endometriosis generally had high positive predictive value. However, both sensitivity and negative predictive value were lower than expected because of atypical lesions subsequently diagnosed as endometriosis. Conclusions: These data suggest that when the surgical objective is complete eradication of endometriosis, the surgeon must be prepared to excise all lesions suggestive of endometriosis and tissue atypical in appearance as in most anatomic sites approximately 25% of atypical specimens proved to be endometriosis.”
More Evidence Against Reflux Theory of Origin
Age-stratified Laterality of Endometriosis Does Not Support Reflux Menstruation as the Origin of Endometriosis
Unpublished Scientific Article
MDDavid B Redwine MD
Funding: none
Synopsis
The age-stratified laterality of distribution of endometriosis refutes the peritoneal circulation modification of the theory of reflux menstruation as the origin of endometriosis.
Abstract
Objective: To tabulate the frequencies of involvement of left and right sided uterosacral and ovarian endometriosis in age-group intervals.
Design: Retrospective review of contemporaneously-tabulated data.
Setting: Tertiary international referral practice specializing in the surgical treatment of endometriosis.
Patients: All patients with no previous surgical treatment of endometriosis.
Interventions: Pelvic mapping of anatomic sites involved by endometriosis.
Outcome measures: Tabulation of unilateral uterosacral and ovarian disease.
Results: There was a small and mostly statistically insignificant predominance of left sided lesions that did not diverge with increasing age.
Conclusions: The incidence of left-sided disease did not increase with advancing age as would be predicted by the peritoneal circulation modification of Sampson’s theory of origin of endometriosis.
Introduction
The origin of endometriosis has been the subject of intense speculation for decades. Sampson’s theory of reflux menstruation is favored by many researchers and clinicians. This theory asserts that during menstruation, viable endometrial cells or small endometrial tissue fragments pass from the uterine cavity, through the fallopian tubes, and exit the fimbriated ends of the tubes into the peritoneal cavity. Once these viable cells enter the peritoneal cavity, three sequential steps occur: 1. Viable cells or tissue fragments must attach to intraperitoneal surfaces; 2. These cells or tissue fragments must then proliferate and invade the surfaces to which they have attached; 3. The autotransplant disease called endometriosis becomes established. There is universal agreement that the only acceptable scientific proof of this theory would be robust photomicrographic evidence of the first two steps occurring in vivo. Since such required scientific proof of Sampson’s theory is absent, support for this theory comes from circumstantial evidence.
A recent argument offered as circumstantial evidence of the theory of reflux menstruation is the peritoneal circulation modification of Sampson’s theory. This modification is based on mapping of the frequency of involvement of anatomic sites by endometriosis, such as the uterosacral ligaments and ovaries (1,2,3,4), ureter (5), intestines (6), and sciatic nerve (7). In this type of study, it has been observed among women with surgically diagnosed endometriosis that when only one uterosacral ligament or one ovary is involved, the left side is more commonly involved than the right side, whereas the right sciatic nerve is involved more commonly than the left. It is argued that these differences in laterality of involvement of pelvic structures are due to the alteration of a clockwise flow (as an observer faces a patient) of peritoneal fluid by the sigmoid colon. In this model, a putative peritoneal circulation of fluid descending along the left abdominal wall is directed medially by the presence of the sigmoid colon. Endometrial cells or tissue fragments entering the pelvis via reflux menstruation from the left fallopian tube are thus alleged to remain in a protected microenvironment, trapped in an eddy lateral to the sigmoid colon. This allows these cells to remain in contact with the left side of the pelvis for a longer length of time since they not swept away by the current, resulting in an increased likelihood of attachment, proliferation and invasion and subsequent development of endometriosis on the left side of the pelvis. Each month, when reflux menstruation takes place, the left side of the pelvis is preferentially seeded by the refluxed cells or tissue fragments which are allowed to remain in contact with pelvic surfaces for a longer length of time. The end result of this model is that the left uterosacral ligament and left ovary are more commonly involved than their contralateral twin structures.
It is further argued that the sigmoid colon simultaneously protects the area of the roots of the sciatic nerve on the left side, thus protecting them from reflux menstruation. This concept of an organ simultaneously promoting and inhibiting the occurrence of a disease on the same side of the body is unique in medicine. Since the area of the right sciatic nerve roots is not protected by the sigmoid colon, endometrial cells or tissue fragments refluxed from the right tube can attach to the area of these nerve roots, thus explaining the greater frequency of right-sided sciatic nerve endometriosis according to this theory.
The pelvic mapping studies offered in support of the peritoneal circulation modification of Sampson’s theory have looked at the cumulative frequency of involvement of pelvic sites of interest in populations of women with endometriosis. Most studies have concentrated on the unilateral involvement of these sites, especially the uterosacral ligaments and ovaries.
There is more information to be obtained from pelvic mapping than such simple overall observations of the distribution of unilateral disease in a large group of women.
Age-stratified pelvic mapping would allow new insight into the validity of the pelvic circulation/sigmoid obstruction modification of Sampson’s theory. If with every menstrual flow new endometrial cells were deposited and preferentially allowed to attach and propagate into lesions of endometriosis, then as women get older the left side of the pelvis should be progressively more commonly diseased than the right side of the pelvis. As a result, as older age groups of women with endometriosis are examined, the difference between left and right-sided pelvic involvement should progressively increase. This paper examines the frequency of unilateral endometriosis involving the uterosacral ligaments and ovaries by five-year age intervals to determine if a significant difference of laterality of involvement exists and to determine if this difference progressively increases with advancing age.
Materials and Methods
The computerized database of the Endometriosis Institute of Oregon contains detailed, prospectively-obtained information on over 2,800 women with surgically-diagnosed endometriosis operated on by the senior author (DBR). This is the largest such database in the world, and the details of its management have been described in previous publications (8,9,10).
In addition to administrative information on each patient such as name, birthdate, address, etc, an important component of each patient record is mapping of sites of involvement by biopsy-confirmed endometriosis. Excision of endometriosis has been performed exclusively, with all reported visual manifestations of endometriosis subject to aggressive excision. The pelvic and intestinal sites of involvement by biopsy-proved endometriosis were then entered into fields in the database according to individual anatomic sites of involvement as previously described (11). Institutional review board approval for this study was not sought since pelvic mapping did not affect patient treatment, did not violate patient confidentiality, and is an extension of the revised American Fertility Society classification system of endometriosis, which is a recommended routine part of patient record-keeping.
The database was queried for patients with no previous surgical therapy who had been found to have histologically documented endometriosis of the uterosacral ligaments and ovaries. Patients were stratified into five-year age intervals.
Statistical analysis was performed using McNemar’s chi-square analysis to accept or exclude the null hypothesis that there is no significant difference in laterality of disease for each age interval. A significance level of p<0.05 was chosen in order to match most previous publications on this topic. A significance level of p>0.05 would therefore indicate that the difference in involvement of a left sided pelvic structure versus its twin on the right side was not statistically significant. Also, for each age interval, the amount (positive or negative) by which the frequency of left sided disease exceeded that of the right side was observed to see if it remained positive across all age groups and increased progressively across advancing age groups, which would be an inevitable result of the peritoneal circulation modification of Sampson’s theory.
Results
A total of 2841 patients were in the database, 1471 of whom had no previous surgical therapy for endometriosis or pelvic pain. Of these, 845 patients had biopsy proven endometriosis involving either one or both uterosacral ligaments. Overall, 224 had only left sided disease, and 165 had only right sided disease, with 456 having bilateral disease of the uterosacral ligaments.
When patients with unilateral disease of the uterosacral ligaments were stratified into eight five-year age intervals (Table 1), five of the eight age intervals had disease more commonly found on the left side, although this finding was statistically significant only for the 25 – 29 year age interval. When the chi square analysis was performed for the entire group of patients with uterosacral ligament disease, the chi square level was 8.95, p <0.005. However, this significance for the entire population was due entirely to the effect of the findings in the 25 – 29 year age interval. The difference between the frequencies of left and right sided disease of the uterosacral ligaments did not increase sequentially with, instead varying positively or negatively among the age groups.
Four hundred and three patients had biopsy proven endometriosis of the ovaries, with 130 involving the left ovary only, 122 the right ovary only, and 151 with bilateral ovarian endometriomas, a non-significant difference in laterality. Four of the eight age group intervals had ovarian disease more commonly found on the left side than on the right. None of these differences was statistically significant, and an increasing frequency of involvement of the left ovary vs the right ovary was not seen with advancing age. As with uterosacral ligament involvement, the differences between the frequencies of left and right-sided ovarian endometriosis varied positively and negatively across the age intervals and did not show a progressive increase with age as the peritoneal circulation model predicts.
Discussion
The peritoneal circulation modification of Sampson’s theory of origin of endometriosis is refuted by these findings. The laterality of involvement of pelvic structures cannot be used as circumstantial evidence for Sampson’s theory.
Many authors have described trends related to laterality of endometriosis lesions involving various organs and structures. The slight left-sided predominance has been championed as strong circumstantial evidence in favor of Sampson’s theory. These authors commonly cite studies which they believe establish the existence of a peritoneal circulation that supposedly runs clockwise as an observer looks at a patient. If this notion were true, then each month with the menstrual flow, endometrial cells or tissue fragments should preferentially accumulate in areas protected from this circulation, such as the left side of the pelvis which allegedly is protected by the sigmoid colon. Since this process is argued to begin with menarche, progressively older age groups of women should have progressively more disease in those protected areas. This is because each month refluxed cells are preferentially allowed to remain in longer contact with pelvic surfaces in such a protected area, allowing the cells an increased opportunity to attach and then to proliferate and invade. With each passing menstrual flow, as more disease accumulates in such a susceptible protected location, the difference in frequency of involvement of the left vs right side of the pelvis should increase as older age groups are examined. Our results indicate clearly that this does not occur.
Our age-stratified results forcefully contradict the notion that the frequency of involvement of the left side of the pelvis is statistically different from the right side, since such statistical significance was found in only one age group of patients with unilateral uterosacral ligament disease and in none of the age groups with ovarian disease. Additionally, our results indicate that an increasing frequency of involvement of the left side of the pelvis does not occur with advancing age, a direct refutation of one of the inevitable predictions of the simplistic peritoneal circulation modification of Sampson’s theory. These results were revealed by age-stratified analysis of patients with endometriosis, illustrating the imprecision and misleading results which can result from collapsing all data into one large data set as has been done with most previous papers.
One other paper presented results with findings similar to ours. Bazi et al (3) found that patients less than 35 years of age had a statistically significant predilection of endometriomas for the left ovary than patients 35 and older, although the numerical basis for their calculation was not presented and patients less than 26 years of age were not included in the analysis.
A two-part question arises in considering the alleged peritoneal circulation: What evidence has been published which supports the existence of this peritoneal circulation in the first place, and does this evidence support the contention of champions of the peritoneal circulation modification of Sampson’s theory? The literature was searched for papers which might shed light on the existence of the type of peritoneal circulation alleged to exist by supporters of the peritoneal circulation modification of Sampson’s theory.
Mitchell (12) studied the patterns of spread of intraperitoneal fluid in a rather unphysiologic way. Working on stillborn infants, artificial perforations were created in the intestinal tract and barium was injected in to the intestines over three hours, during which x-rays were taken. Depending on where the perforation was created, barium could flow literally in any direction. In one female stillborn, he injected barium into the uterus at 100 mm Hg pressure and noted that the barium flowed from the right tube up the right colonic gutter toward the right subphrenic space. He mentioned this single instance of intrauterine injection in his paper, but did not include this in his discussion, since he considered this single observation to be unphysiologic due to the high pressure of injection. The methodology in this paper is unphysiologic and cannot be accepted as what happens in living adult females. However, if the evidence observed during the single case of intrauterine injection were to be accepted as valid, it would predict that involvement of the right colonic gutter and right upper quadrant would be more frequent than involvement of the right hemipelvis, and this does not occur.
Drye (13) found that in the standing position, the hydrostatic pressure in the bottom of the human pelvis was higher than the hydrostatic pressure in the epigastrium as measured by balloon catheters. When subjects were upright, this pressure difference remained constant, other than slight respiratory variations. This pressure difference went away when the subjects were supine. These results are unsurprising and straightforward manifestations of basic physics. The increased pressure in the pelvic region in the standing position is not evidence of a peritoneal circulation, nor would it force fluid out of the pelvic region into the upper abdomen any more than fluid in the bottom of a glass would be forced to the surface by the pressure difference alone. The unvarying difference of pressure between the upper abdomen and lower abdomen is proof that a circulation does not exist in the upright position.
Meyers in 1970 (14) studied the distribution of radioopaque dye injected into the peritoneal cavity in 20 patients with intraperitoneal effusions and abscesses. Dye was injected into the abdomen through the left upper quadrant. This was done in various positions on a tilt table but not in the upright position. Fluoroscopy revealed the dye to initially sink into the pelvis, then rise up both colonic gutters as the table was tilted, with more pronounced upward flow on the right than the left. The fluid ascending the right gutter pooled preferentially in Morrison’s pouch beneath the liver, from where it could then drift into the sub-phrenic space on the right. This study by Meyers was rather unphysiologic since patients had intraperitoneal pathological conditions, and the patient’s position was changed at will on a tilt table. Also, the observations during the short duration of a radiological exam would not necessarily be an accurate indication of what happens over several years in vivo. For these reasons, this study cannot be accepted as evidence of a natural peritoneal circulation as envisioned by supporters of the peritoneal circulation modification of Sampson’s theory. However, for the sake of argument, if this study by Meyers were accepted as proving that a natural peritoneal circulation exists, then it could not be characterized as clockwise since fluid flowed up the left colonic gutter also. The fact that dye ascended from the left side of the pelvis up the left paracolic gutter means that there is not a “protected microenvironment” on the left side of the pelvis due to the presence of the sigmoid colon. Therefore, the inference that the sigmoid colon offers a protected environment on the left side of the pelvis is false, based on Meyers’ findings. If the peritoneal circulation modification of Sampson’s theory were true, there should be some substantial involvement of the left colonic gutter in the lower abdomen due to the flow upward along the left pelvic and abdominal sidewall, but disease in that area is rare. Also, involvement of Morrison’s pouch by endometriosis would be more common than diaphragmatic disease since particles of endometrium should be carried there first, yet involvement of Morrison’s pouch by endometriosis is extremely rare, if in fact it has ever been reported. Therefore, sites of pelvic and abdominal involvement by endometriosis are not accurately predicted by the patterns of artificially induced peritoneal flow described in this study.
A subsequent study by Meyers (15) correlated sites of metastatic cancer with the flow of intraperitoneal dye. Again, dye was injected into the abdomen and x-rays were taken in multiple positions, including Trendelenberg and oblique views. Up to 200 mL of dye was injected, and the hypertonicity of the dye induced mild ascites, which may have altered the results. The dye collected in the cul-de-sac, the termination of the small bowel mesentery (terminal ileum), the superior aspect of the sigmoid mesocolon, and the right paracolic gutter. These findings were correlated with metastatic implants from ovarian, pancreatic, gastric, and colon cancer. Not surprisingly, among 15 patients with ovarian cancer (the closest approximation to what is alleged to result from reflux menstruation) nine had seeding of the cul-de-sac, 7 had seeding of the small bowel mesentery near the ileocecal junction, 1 had seeding of the medial aspect of the sigmoid mesentery, and 4 had seeding of the right colonic gutter. Once again, the nature of the investigation was unphysiologic due to the tilting of the table during the short duration of the exam, and no clockwise circulation of fluid was expressly described, so this paper cannot be accepted as evidence of a clockwise circulation. However, if the results of this study were to be accepted as predicting where endometriosis would likely occur by virtue of Sampson’s theory, endometriosis should occur preferentially in the cul-de-sac, small bowel mesentery, medial sigmoid colon, and right colonic gutter, following the patterns seen with ovarian cancer. While endometriosis is most commonly found in the cul-de-sac, it rarely involves the small bowel mesentery near the ileocecal junction, and involvement of the medial sigmoid colon and right colonic gutter are uncommon. Once again, sites of pelvic and abdominal involvement by endometriosis are not accurately predicted by the patterns of peritoneal flow described in this study.
Rosenshein et al (16) conducted a study in anesthetized rhesus monkeys where two catheters were inserted into the abdominal cavity. One catheter was inserted in the right upper quadrant and advanced down into the pelvis. The second catheter was inserted in the left upper quadrant and not advanced. Radioactive albumen was injected through the left upper quadrant catheter in a volume of 250 mL in three animals and 10 mL in two animals. Over the course of almost three hours of observation, various maneuvers were applied to the monkeys, including abdominal massage for five minutes, and various position changes including 30 degree Trendelenburg and reverse Trendelenburg positions. The fluid was noted to settle in the cul-de-sac and eventually track up the course of the right-sided catheter when 250 mL was injected, with less pronounced distribution of the smaller volume of fluid. This finding is unsurprising since it demonstrates that fluid will take the path of least resistance, unphysiologic since menstruating women don’t usually have such catheters in place and probably don’t have 250 mL of refluxed menses in their peritoneal cavity, and therefore unhelpful as evidence supporting either a clockwise peritoneal circulation or Sampson’s theory. The authors of this study said nothing about a clockwise circulation existing in the peritoneal cavity of rhesus monkeys.
Rosenshein et al, (17) in a discussion of the diffusion of radiocolloids in the peritoneal cavity describes the actions of the diaphragm as a ‘pump, draining the peritoneal fluid upwards with each inspiration.’ There is nothing in the context of the rest of this paper which would lead anyone to conclude that there is a clockwise or counterclockwise current in the abdominal cavity.
Foster et al (18) published a paper commenting on the right-sided predilection of thoracic endometriosis. Citing Rosenshein et al (17), they presented a schematic diagram of a clockwise circulation of peritoneal fluid, although they presented no evidence of their own to suggest that this circulation existed. This schematic diagram, a crude and incomplete explanation of Rosenshein’s findings, cannot be accepted as scientific evidence of a clockwise peritoneal circulation.
McQueen et al (19) placed 1.5 mL of radionuclide in the vaginas of 23 women without endometriosis and found that after one hour, the material had collected in the cervix, uterus, and around the adnexae bilaterally. A peritoneal circulation was not being sought and such a circulation was not mentioned in this paper.
From this review of the literature it is clear that there is no scientific evidence of the existence of a clockwise peritoneal circulation which is alleged to exist by proponents of the peritoneal circulation modification of Sampson’s theory. Supporters of this theory do not cite any reference with scientific evidence of this circulation. Any arguments which are based on the alleged existence of such a circulation are therefore without foundation.
The peritoneal circulation modification of Sampson’s theory is overly simplistic and does not address fundamental questions which arise from it. For example, is the alleged peritoneal circulation a stronger force on the tissue fragments than that exerted by surface tension of moist surfaces? Are the effects of gravity stronger than the alleged circulation?
The statistics which have been used to support the peritoneal circulation modification of Sampson’s theory are inconsistent with our findings and occasionally internally contradictory. Most of the published papers on the laterality of endometriosis have focused on the cumulative frequencies of involvement of the uterosacral ligaments and ovaries, since these are distinctly lateral structures about which there would be no confusion over assignment of laterality. Most of the papers on the subject have examined the relative left vs right laterality of involvement of paired pelvic structures by McNemar’s chi square test (although this was not specifically mentioned in the Methods sections of these papers.). The schematic formula for this statistic is (L – R)2/T, where L is the number of patients with left-sided unilateral disease and R is the number of patients with right-sided unilateral disease and T is the sum of L + R.
One paper published in support of the peritoneal circulation modification of Sampson’s theory actually contains statistical evidence refuting that theory. Parazzini, et al, (20) wrote a paper comparing the incidence of endometriosis in the left vs. right hemipelvis. Rather than using McNemars’s chi square test (which was used in all other papers published previously on this subject by members of this writing group and numerous other authors) to analyze their data, they instead used the binomial distribution and Poisson’s approximation to measure the significance of their findings that the left side was more commonly involved than the right side. However, when the traditionally-used McNemar’s chi-square test is applied to their data, the chi-square value is 2.58 (p>0.1) which is not significant. Thus, this paper actually refutes its authors’ claims when the correct statistical test is run. In order for a theory to be valid, it should be consistently supported by the same statistical test used to support it in other publications rather than searching for a different statistical test which might give support.
Papers championing the peritoneal circulation modification of Sampson’s theory have avoided the question of the large percentage of patients with bilateral disease. For a theory to be valid, it must include an explanation for all observations, not just a selected portion such as those with only unilateral disease.
Reflux menstruation itself as a theory of origin of endometriosis remains unproven, supported only by many lines of circumstantial evidence. The peritoneal circulation modification of this theory has introduced a new, strained attempt to support this theory by circumstantial evidence. If such a peritoneal circulation were to exist, our findings would suggest that this circulation reverses direction at various times in a woman’s lifespan, then eventually slows. Such variation of an unproven circulation seems unlikely and has not been proposed by supporters of this concept.
The peritoneal circulation modification of Sampson’s theory of reflux menstruation as the origin of endometriosis has resulted in an unusual situation in scientific publication. A long-standing but scientifically unproven theory of origin (Sampson’s theory) of an important disease seeks support from inconsistent and contradictory statistical evidence of laterality of disease which infers the physical results of a peritoneal circulation which has not been scientifically proven to exist. Since the peritoneal circulation modification of Sampson’s theory is based on a scientifically unproven fiction, it should be abandoned.
Summary
There is a slight left sided predominance of endometriosis involving the uterosacral ligaments and ovaries. The difference between the frequency of left-sided involvement and right-sided involvement does not increase with advancing age. The frequency of involvement of the left side of the pelvis does not increase linearly with advancing age. A clockwise circulation of peritoneal fluid has not been scientifically proven to exist. Supporters of the peritoneal circulation modification of Sampson’s theory have selected portions of studies which support their contentions and have ignored those portions that do not. The laterality of distribution of endometriosis does not support Sampson’s theory via the peritoneal circulation model. Future papers on the theory of reflux menstruation as the origin of endometriosis should focus on the most easily obtainable scientific evidence of all: robust photomicrographic evidence of the in vivo occurrence of the two missing steps of this theory, adhesion and implantation of the endometrial cells at their final resting sites within the pelvis.
References
Vercellini P, Busacca M, Aimi G, Bianchi S, Frontino G, Crosignani PG. Lateral distriburion of recurrent ovarian endometriotic cysts. Fertil Steril 2002;77:848-9.
Vercellini P, Aimi G, De Giorgi O, Maddalena S, Carinelli S, Crosignani PG. Is cystic ovarian endometriosis an asymmetric disease? Br J Obstet Gynaecol 1998;105:1018-21.
Bazi T, Nader KA, Seoud MA, Charafeddine M, Rechdan JB, Zreik TG. Lateral distribution of endometriomas as a function of age. Fertil Steril 2007;87:419-21.
Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol 2003;101:164 – 6.
Vercellini P, Pisacreta A, Pesole A, Vicentini S, Stellato G, Crosignani PG. Is ureteral endometriosis an asymmetric disease? Br J Obstet Gynaecol 2000;107:559 – 61.
Vercellini P, Chapron C, Fedele L, Gattei U, Daguati R, Crosignani PG. Evidence for asymmetric distribution of lower intestinal tract disease. Br J Obstet Gynaecol 2004;111:1213-7.
Vercellini P, Chapron C, Fedele L, Frontino G, Zaina B, Crosignani PG. Evidence for asymmetric distribution of sciatic nerve endometriosis. Obstet Gynecol 2003 102:383-7.
Redwine DB. Database management for personal computers, I. J Am Assoc Gynecol Laparosc 1996; 3: 469-74.
Redwine DB. Database management for personal computers, II. J Am Assoc Gynecol Laparosc 1996; 3: 643-50.
Redwine DB. Database management for personal computers, III. J AmAssocGynecol Laparosc 1996; 4: 95 – 101.
Redwine, DB. The distribution of endometriosis in the pelvis by age groups and fertility. Fertil Steril 1987; 47:173-175
Mitchell GAG. The spread of acute intraperitoneal effusions. Br J Surg 1941;28:291 – 313.
Drye JC. Intraperitoneal pressure in the human. Surg Gynecol Obstet 1948;87:472-5.
Meyers MA. The spread and localization of acute intraperitoneal effusions. Radiology 1970;95:547 – 554.
Meyers MA. Distribution of intra-abdominal malignant seeding: Dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med 1971;119:198 – 206.
Rosenshein N, Blake D, McIntyre PA, Parmley T, Natarajan TK, Dvomicky J, et al. The effect of volume on the distribution of substances instilled into the peritoneal cavity. Gynecol Oncol 1978;1:106 – 10.
Rosenshein NB, Leichener PK, Vogelsang G. Radiocolloids in the treatment of ovarian cancer. Obstet Gynecol Surv 1979;34:708 – 20.
Foster DC, Stern JL, Bescema J, Rock JA, Woodruff JD. Pleural and parenchymal pulmonary endometriosis. Obstet Gynecol 1981; 58:552 – 6.
McQueen D, McKillop JH, Gray HW, Callaghan M, Monaghan C, Bessent RG. Radionuclide migration through the genital tract in infertile women with endometriosis. Hum Reprod 1993;8:1910 – 4.
Parazzini F, et al. Left:right side ratio of endometriotic implants in the pelvis. Eur J Obstet Gynecol 111(2003) 65-67.
WEBLINK TO ARTICLE http://pacificendometriosis.com/age-stratified-laterality-of-endometriosis-does-not-support-reflux-menstruation-as-the-origin-of-endometriosis/

